In the ever-evolving landscape of aesthetic technology, SEA HEART GROUP has once again made headlines with the successful FDA approval of their latest innovation, the Model SH-VD910 Diode Laser Hair Removal Machine. This cutting-edge device represents a significant milestone in the field of laser hair removal, offering unmatched precision, safety, and efficacy. Let's delve into the details of this groundbreaking development and explore why it has garnered such attention in the industry.
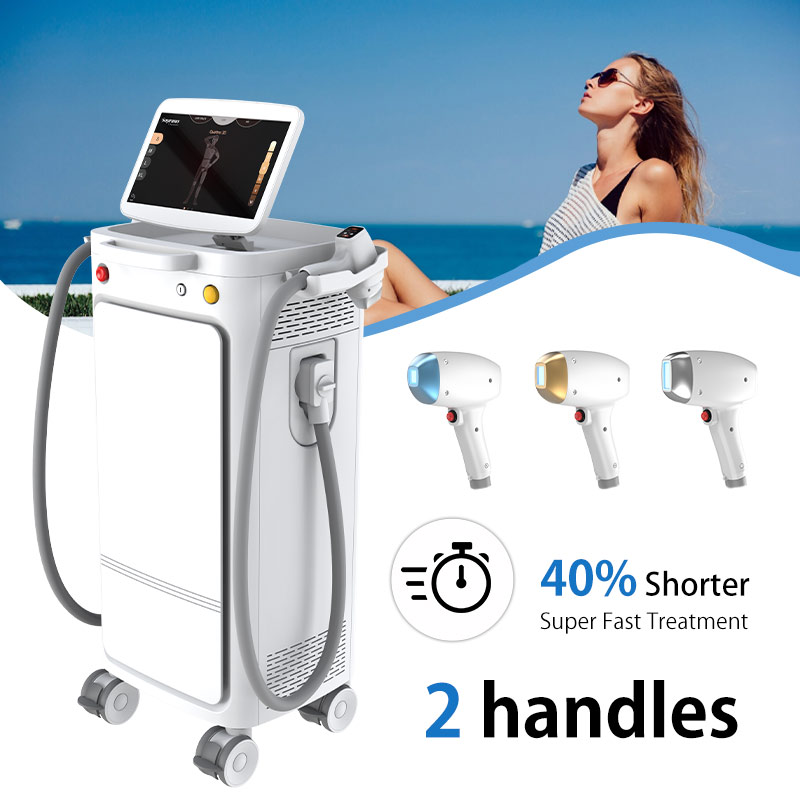

The Evolution of Laser Hair Removal Technology
To understand the significance of the SH-VD910, it's essential to trace the evolution of laser hair removal technology. Traditional methods often pose challenges in terms of efficacy, safety, and patient comfort. However, advancements in diode laser technology have revolutionized the landscape, offering a superior solution for permanent hair reduction. The SH-VD910 builds upon this foundation, incorporating state-of-the-art features to deliver exceptional results.
Key Features and Benefits of the SH-VD910
The SH-VD910 boasts a plethora of features that set it apart from conventional laser hair removal devices. Its advanced diode laser technology targets hair follicles with unparalleled precision, effectively inhibiting hair growth while minimizing discomfort for the patient. Furthermore, the device's ergonomic design and feelingsive interface ensure ease of use for practitioners, enhancing efficiency and patient satisfaction. With adjustable settings and customizable treatment protocols, the SH-VD910 offers versatility to address diverse patient needs and skin types.
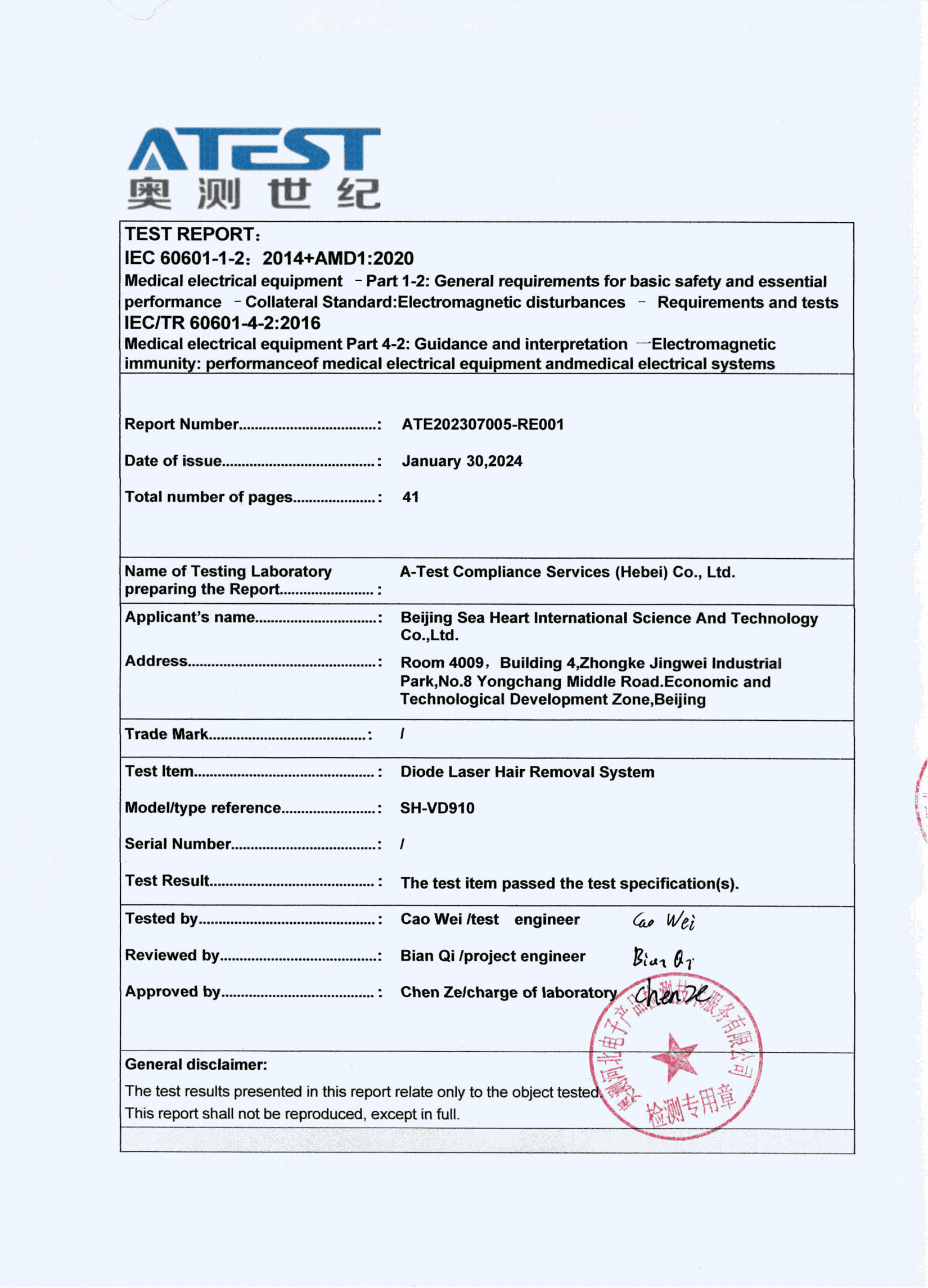

FDA Approval and Regulatory Compliance
One of the most significant milestones for any medical device is obtaining regulatory approval from governing bodies such as the FDA. The SH-VD910 has undergone rigorous testing and evaluation to meet the stringent standards set forth by regulatory authorities. Its successful FDA approval reaffirms its safety and efficacy, providing healthcare professionals and patients alike with confidence in its performance. This endorsement further solidifies SEA HEART GROUP's commitment to upholding the highest standards of quality and compliance.
Clinical Evidence and Patient Outcomes
The efficacy of the SH-VD910 is supported by a robust body of clinical evidence and real-world patient outcomes. Clinical studies have demonstrated significant reductions in hair regrowth following treatment with the device, with many patients achieving long-lasting results after a series of sessions. Moreover, patient satisfaction rates are consistently high, with individuals praising the device's effectiveness and minimal side effects. These compelling outcomes underscore the SH-VD910's status as a game-changer in the field of laser hair removal.
The Future of Aesthetic Technology
As technology continues to advance, the future of aesthetic treatments looks brighter than ever. The SH-VD910 represents just one example of the innovative solutions emerging in the industry, offering hope for individuals seeking safe, effective, and long-lasting hair removal options. With ongoing research and development efforts, SEA HEART GROUP remains at the forefront of this transformative journey, driving progress and pushing the boundaries of what's possible in aesthetic medicine.

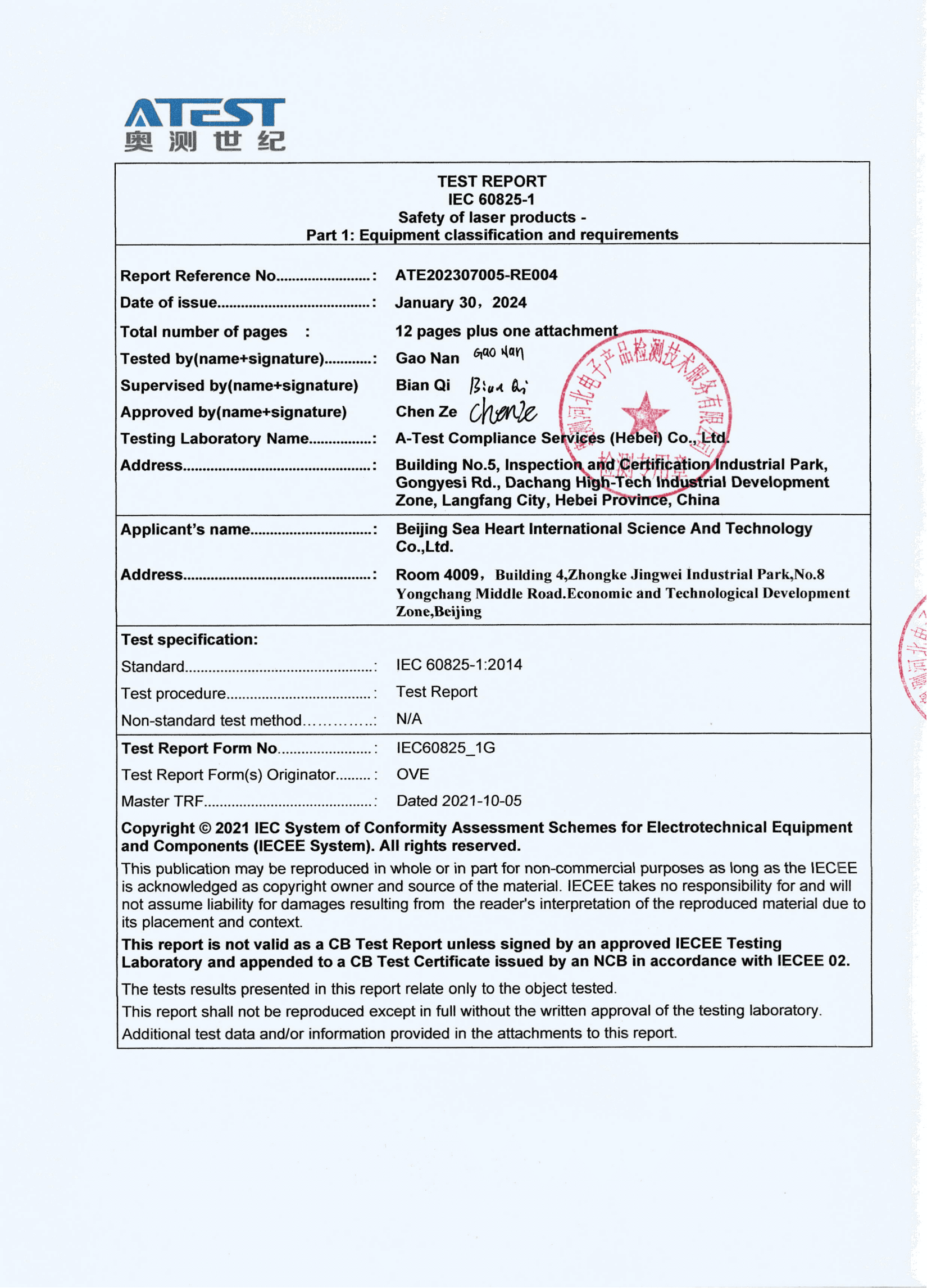
Conclusion:
In conclusion, the FDA approval of the Model SH-VD910 Diode Laser Hair Removal Machine marks a significant milestone in the field of aesthetic technology. With its advanced features, proven efficacy, and regulatory endorsement, this innovative device is poised to revolutionize the landscape of laser hair removal. As SEA HEART GROUP continues to push the boundaries of innovation, patients can look forward to safer, more effective, and more accessible aesthetic treatments in the years to come.






 Mastering HIFU: Step-by-Step Operating Guide and Real-Life Demonstrations
Mastering HIFU: Step-by-Step Operating Guide and Real-Life Demonstrations
 Understanding the Differences Between Picospeak 300 and 500 in One Article
Understanding the Differences Between Picospeak 300 and 500 in One Article
 Russian Agents Experience Warm Welcome and Cutting-Edge Beauty Instrument Exports at SEA HEART GROUP
Russian Agents Experience Warm Welcome and Cutting-Edge Beauty Instrument Exports at SEA HEART GROUP